Oxafence Active Protection™
Enabling antimicrobial performance in materials.
Oxafence is Singletto’s materials-applied technology platform, with performance demonstrated in regulated applications and designed for integration into existing products.
Why a New Approach is Needed
Many products people expect to exist simply do not – not because the need isn’t clear, but because available antimicrobial technologies have not been able to support them.
For example, surveys consistently show that a large majority of people believe virus inactivation would be an important feature in face masks. Yet until recently, masks with this capability were largely absent – not due to lack of demand, but because existing antimicrobial approaches could not be effectively integrated into mask materials.
Across categories, traditional antimicrobial strategies often rely on chemistries that are passive, depleting, or constrained by durability, resistance, or environmental considerations. As a result, entire classes of active materials have remained out of reach.
Oxafence was developed to unlock these categories by enabling antimicrobial performance directly within materials, making new kinds of products possible where they previously were not.
What Makes Oxafence Different
A materials-applied approach to antimicrobial performance
Active, not passive
Oxafence is designed to support ongoing antimicrobial performance rather than one-time or surface-limited effects.
Applied to materials
The technology integrates into existing materials and manufacturing processes, rather than requiring new product categories.
A platform, not a single use
Oxafence is designed for application across multiple environments where traditional antimicrobial approaches have struggled.
Scientific Foundation
Grounded in frontline clinical research
Oxafence originated from surgeon-led research conducted during the COVID-19 pandemic, including WHO task force-organized studies focused on clinically relevant mask-based assays examining real-world pathogen inactivation. These studies were conceived and led by Singletto co-founder surgeons James Chen, MD, and Thomas Lendvay, MD, based on an original hypothesis developed in response to unmet clinical needs.
Findings from this work and subsequent studies were published in leading peer-reviewed clinical journals, including Infection Control & Hospital Epidemiology (ICHE) and the American Journal of Infection Control (AJIC). This research laid the scientific foundation for Oxafence Active Protection™ and its materials-applied approach.
This body of work, which spans FDA-cleared products, WHO task force-organized research, and publication in leading peer-reviewed infection control journals, reflects a level of scientific and regulatory scrutiny uncommon for materials-applied antimicrobial technologies.
Where Performance Matters
Designed for environments where microbial exposure accumulates
In many critical environments, materials are used continuously, shared widely, and exposed repeatedly to bacteria and viruses. Over time, microbial exposure accumulates on materials, yet most are designed only to act as passive barriers, not to address what they encounter.
Oxafence was developed for environments where bioburden builds during real-world use, and where material performance matters most.
Healthcare environments
In clinical settings, materials are worn or handled across long shifts and multiple interactions, where exposure to bacteria and viruses is unavoidable and cumulative.
Military & operational use
Operational environments demand solutions that perform under sustained exposure and real-world conditions, without added complexity or changes to how equipment is used.
Shared & everyday settings
As materials increasingly intersect with dense, shared use – in workplaces, transit, and public spaces – new approaches are needed to account for ongoing microbial exposure.
In Practice: Masks
The first commercial application of Oxafence Active Protection™ is in FDA-cleared face masks (K231741), where the technology is applied to mask materials during manufacturing.
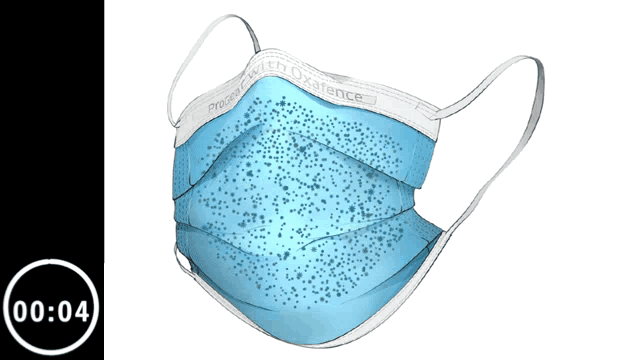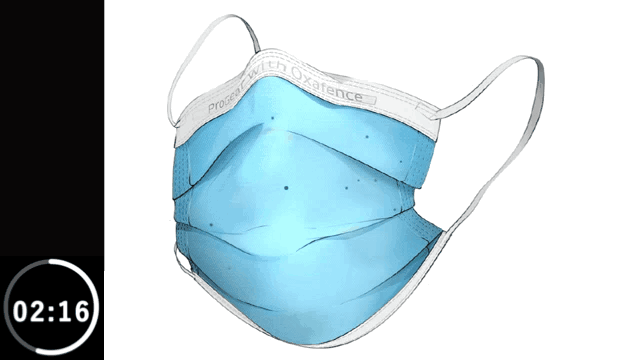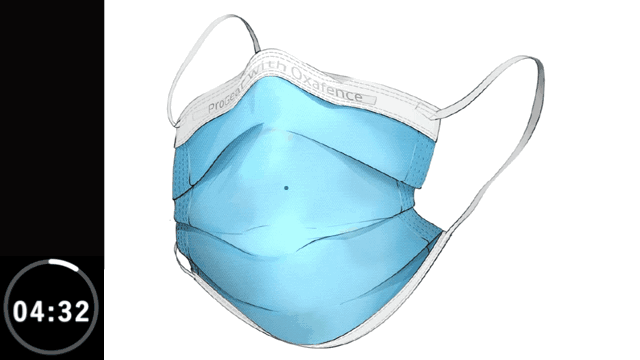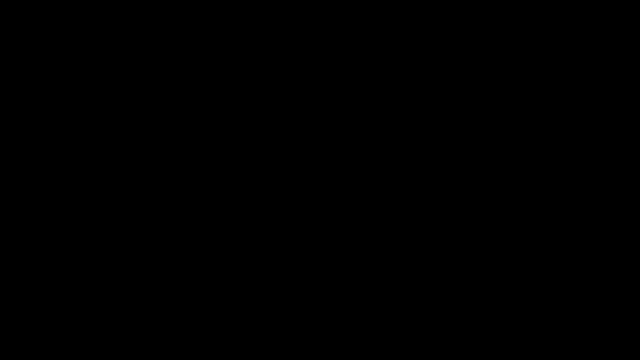
Inactivates 99.9% of tested viruses within 5 minutes on the outer surface, including H1N1 Influenza and OC43 human Coronavirus.*
Animation is illustrative and time-compressed. It reflects laboratory test results for H1N1 Influenza obtained from independent ISO-accredited laboratories evaluating the Oxafence ProGear mask, including observations at both 1-minute and 5-minute time points, shown over a compressed 5-minute interval.
* The ProGear Surgical Mask with Oxafence inactivates single isolates of the following test viruses after five minutes of contact with the treated surface of the face mask in laboratory (in vitro) tests under a lighting intensity of 500 ± 25 lux: Influenza A/PR/8/34/H1N1 (TC adapted, ATCC VR-1469), 3 log or 99.9% inactivation; Betacoronavirus 1 OC43 (ATCC VR-1558), 4 log or 99.99% inactivation; Feline Calicivirus F-9 (ATCC VR- 782), 4 log or 99.99% inactivation. Antiviral efficacy on other viruses has not been evaluated. Correlations between in vitro testing results and any clinical event have not been evaluated.
Partnerships
Designed to scale through partnership
Singletto works with manufacturing, research, and distribution partners to integrate Oxafence Active Protection™ into materials across current and future applications.